The Fuel and Engine Bible - how engines work including 2 stroke, 4 stroke and wankel (rotary) engines, fuel, octane rating, power, bhp, gas types and grades, carburettors, fuel injection, tuning, tweaking, nitrous, turbos, superchargers, chipping, hybrids, how to keep your engine running at peak fitness and much more.
The Fuel & Engine Bible
And so to fuel (or gasoline or petrol)
Petrol (or gasoline if you're American) is a distilled and refined oil product made up of hydrogen and carbons - a hydrocarbon. A long-chain hydrocarbon to be exact (so don't get it on your skin - its carcinogenic). It's designed to be relatively safe to handle, if you're careful. ie. it doesn't spontaneously combust without extreme provocation. When you have a petrol fire, it's not the petrol itself that is burning, it's the vapour, and this is the key to fueling an engine. The carburettor or fuel injectors spray petrol into an air stream. The tiny particles of petrol evaporate into a vapour extremely quickly, and combined in a cloud with the air, it becomes extremely combustible. The smaller the particles from the carburettor jet or fuel injector, the more efficiently the mixture burns.
Detonation, pre-ignition, pinking, pinging and knocking.
Remember I said petrol doesn't spontaneously combust? Well it can if the conditions are right, and the conditions are extreme heat and pressure - exactly the conditions you find in the combustion chamber. When this happens, it's called detonation or pre-ignition. Diesel engines rely on this process because they don't have a spark plug in the traditional sense of the word. However in petrol engines, when this happens (also known as dieseling), it's a Very Bad Thing. Engines are designed to have the fuel-air mix burn at a fixed point in the cycle, not explode randomly. Whilst it might look like an explosion, if you could film it on a super high-speed camera, you'd see the mixture actually burns up very quickly rather than exploding. The video on the right is just that - in-cylinder video of the 4 stroke combustion cycle. The intake valve is on the right, the exhaust valve on the left. Detonation, dieseling or pre-ignition are all terms for what happens when the fuel-air mix spontaneously explodes rather than burning. Normally this happens when the mixture is all fouled up, and the engine is running hot. The temperature and pressure build up too quickly in the combustion chamber and before the piston can reach the top of its travel, the mixture explodes. This explosion tries to counteract the advancing piston and puts an enormous amount of stress on the piston, the cylinder walls and the connecting rod. From the outside of the engine, you'll hear it as a knocking or pinging sound. The precise sound is very hard to describe because every engine sounds slightly different when it happens. But the best way I can describe it is a constant 'toc toc toc' type knocking sound.
Video credit: Original source unknown. Video also available on YouTube and Google Video.
Compression ratio.
The compression ratio of an engine is the measurement of the ratio between the combined volume of a cylinder and a combustion chamber when the piston is at the bottom of its stroke, and the same volume when the it's at the top of its stroke. The higher the compression ratio, the more mechanical energy an engine can squeeze from its air-fuel mixture. Similarly, the higher the compression ratio, the greater the liklihood of detonation.
Octane ratings - how to stop detonation
So you know that a fuel-air mix, given the right conditions, can spontaneously combust. In order to control this property, all petrols have chemicals mixed in with them to control how quickly the fuel burns. This is known as the octane rating of the fuel. The higher the rating, the slower and more controlled the fuel burns.
Put on the geek-shades for a moment and I'll explain octane in more depth. If you don't like being blinded by science, skip down a few paragraphs. For the rest of you, octane is measured relative to a mixture of isooctane (2,2,4-trimethylpentane, an isomer of octane) and n-heptane. An 87-octane gasoline has the same knock resistance as a mixture of 87% isooctane and 13% n-heptane. The octane value of a fuel used to be controlled by the amount of tetraethyl lead in it, but in the 70s and 80s when it became apparent that lead was pretty harmful, lead-free petrol appeared and other substances were introduced to control octane instead.
Measuring octane - RON, MON and the difference between America and the rest of the world.
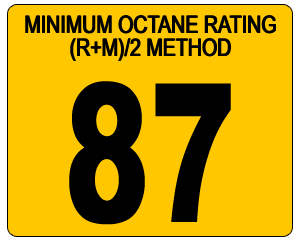
Just so you know, the octane number is actually an imprecise measure of the maximum compression ratio at which a particular fuel can be burned in an engine without detonation. There are actually two numbers - RON (Research octane number) and MON (Motor Octane Number). The RON simulates fuel performance under low severity engine operation. The MON simulates more severe operation that might be incurred at high speed or high load and can be as much as 10 points lower than the RON. In Europe, what you'll see on the petrol pumps is the RON. However, in America, what you'll see on the petrol pump is usually the "mean" octane number - notified as (R+M)/2 - the average of both the RON and MON. This is why there is an apparent discrepancy between the octane values of petrol in America versus the rest of the world. Euro95 unleaded in Europe is 95 octane but it's the equivalent of American (R+M)/2 89 octane.
In America, low altitude petrol stations typically sell three grades of petrol with octane ratings of 87, 89 and 91. High altitude stations typically also sell three grades, but with lower values - 85, 87 and 89.
What factors affect detonation?
There's a bunch of things that can affect how likely an engine is to have detonation problems. The common ones are ambient air temperature, humidity, altitude, your engine's ability to stay cool (ie. the cooling system) and spark timing. Fortunately, nowadays the engine management system of modern cars can compensate for almost all of these by advancing and retarding the ignition timing. This is where the computer slightly adjusts the point in the ignition cycle at which the spark is generated at the spark plug. With older engines that used mechanical points to send current to the spark plugs, adjusting the timing was a manual affair that involved adjusting the distributor cap orientation.
Knock sensors. Most modern cars have knock sensors screwed into the engine at multiple places. These actually detect the vibration or shock caused by detonation (rather than trying to detect the sound) and can signal the engine management system to change the ignition timing to reduce or eliminate the problem.
Octane and altitude
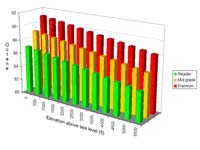
The higher the altitude above sea level, the lower the octane requirement. As a general rule of thumb, for every 300m or 1000ft above sea level, the RON value can go down by about 0.5. For example an 85 octane fuel in Denver will have about the same characteristics as an 87 octane fuel on the coast in Los Angeles. As a practical example of this, I currently live in Salt Lake City which is at around 4,200ft. We travel to Las Vegas from time to time which is at around 2,000ft. Our Subaru has a minimum octane requirement of 89 at sea level - so about 87 where we live. Last time we drove to Vegas, the petrol station we stopped at had run out of 'premium' products so we had to fill up with 85 octane. This, combined with the drop in altitude caused the 'check engine' light to come on because we'd effectively taken the engine from 87 octane at altitude to the equivalent of 83 octane at altitude - way below the minimum required by our car.
The graph here gives a rough idea of how the three main grades of petrol in America perform with respect to octane at altitude.
Octane and power
It's a common misconception amongst car enthusiasts that higher octane = more power. This is simply not true. The myth arose because of sportier vehicles requiring higher octane fuels. Without understanding why, a certain section of the car subculture decided that this was because higher octane petrol meant higher power.
The reality of the situation is a little different. Power is limited by the maximum amount of fuel-air mixture that can be jammed into the combustion chamber. Because high performance engines operate with high compression ratios they are more likely to suffer from detonation and so to compensate, they need a higher octane fuel to control the burn. So yes, sports cars do need high octane fuel, but it's not because the octane rating is somehow giving more power. It's because it's required because the engine develops more power because of its design.
There is a direct correlation between the compression ratio of an engine and its fuel octane requirements. The following table is a rough guide to octane values per engine compression ratio for a carburettor engine without engine management. For modern fuel-injected cars with advanced engine management systems, these values are lowered by about 5 to 7 points.
| Compression ratio | Octane |
|---|---|
| 5:1 | 72 |
| 6:1 | 81 |
| 7:1 | 87 |
| 8:1 | 92 |
| 9:1 | 96 |
| 10:1 | 100 |
| 11:1 | 104 |
| 12:1 | 108 |
The exception to prove the rule. Nowadays, higher octane fuel might actually give you more power but not because of the octane rating itself. Some petrol companies use a denser blend for the higher octane products. Denser blends mean higher energy density per volume (measured in Megajoules per litre - MJ/L). For example:
BP Regular: 32.53MJ/L
BP Premium: 33.08MJ/L
BP Ultimate: 33.28MJ/L
Do these variations in energy density mean you'll get more power out of your engine from premium blends? Yes, but not in a linear fashion - ie. if the premium product has 5% more energy density than the basic product, you won't get 5% more power out of your engine, simply because of the inefficiencies of internal combustion and thermodynamic considerations.
Octane and gas mileage
Here's a good question : can octane affect gas mileage. The short answer is absolutely, yes it can, but not for the reasons you might think. The octane value of a fuel itself has nothing to do with how much potential energy the fuel has, or how cleanly or efficiently it burns. All it does is control the burn. However, if you're running with a petrol that isn't the octane rating recommended for your car, you could lose gas mileage. Why? Lets say your manufacturers handbook recommends that you run 87 octane fuel in your car but you fill it with 85 instead, trying to save some money on filling up. Your car will still work just fine because the engine management system will be detecting knock and retarding the ignition timing to compensate. And that's the key. By changing the ignition timing, you could be losing efficiency in the engine, which could translate into worse gas mileage. Again as a practical example, my little tale above about our trip to Vegas on low octane gas. (Whether you want to believe some bloke on the internet or not is up to you). On the low octane gas on the trip down, we could barely get 23.5mpg out of the Subaru. Once I was able to fill it up again with premium at the recommended octane rating, we got 27.9mpg on the way back. A difference of 4.4mpg over 450 miles of driving.
Doing the maths, you can figure out that by skimping on the price during fill-up, you may save a little money right there and then, but it costs in the long term because you're going to be filling up more often to do the same mileage. My advice? Do what the handbook tells you. After all it's in the manufacturers better interests that you get the most performance out of your car as you can - they don't want you badmouthing them, and in this day and age of instant internet gratification, you can bad-mouth a large company very quickly and get a lot of publicity.
Octane boosters

In some extreme cases, the highest octane fuel available might not solve a knocking or detonation problem. That's normally a symptom of a deeper problem in the engine involving carbon deposits on the cylinder heads, bad spark timing, faulty engine management systems or similar. In these cases, some people choose to add octane booster to their petrol. Basically you fill the tank as normal, then put in a measured amount of octane booster and it further raises the octane level in an attempt to stop the detonation. One of the downsides of this is that it can make the engine harder to start from cold, because the octane booster has made the fuel so much less volatile that it's hard to get it to ignite on the first couple of strokes. Products like Klotz and Redex octane boosters are readily available over the counter in most auto parts stores. Octane boosters are typically used by mis-educated motorcyclists who believe the myth (explained above) that high octane = more power.

Octane boosters tested by Fifth Gear. To try to lay the myth about octane boosters giving more power to bed for once and for all, in 2007 the UK TV show Fifth Gear picked four likely candidates and subjected them to rigorous testing. They picked Nitro Hot Shot, NOS Race Only Octane Booster, Wynn's Power Booster and STP Power Booster. All four products make the usual wild claims about increased gas mileage, more bhp and so on and so forth. They took the products to Oxford Brooks University's engine testing lab. The engine was static-mounted so measurements were made at the flywheel. The throttle was computer controlled so they could reproduce the same scenario over and over again. They first did a baseline test to find out peak bhp with regular unleaded petrol. This involved various constant-throttle settings as well as acceleration and deceleration testing, and a 1-hour constant-speed run to emulate driving on a motorway in clear traffic. Each product was tested using the identical setup, with a 15 minute 'pure' petrol flush being used in between each test to ensure there was no cross-contamination. The results were interesting. Nitrox Hot Shot, NOS Race Only Octane Booster and Wynn's Octane Booster all reduced the overall power by 2bhp. STP Power Booster reduced it by 6%. Now remember this was measured at the flywheel so by the time you induce all the drag of the gearbox and driveline into that equation, you'd likely be looking at a 5% to 10% drop at the wheels. Impressive results for products that claim to increase your engine's power.
In England, octane boosters are typically also sold as "lead replacements" or "4 star additive". A lot of European cars relied on the lead in 4-star petrol for the increased octane. Lower octane unleaded fuels caused a lot of problems when they first appeared, especially with cars that didn't have engine management systems. Knocking and detonation became evident in a lot of cars and for some reason French and German engines were more susceptible than most. Dumping a shot of octane booster in the tank when filling up solved the problem by raising the RON a few points to make it the equivalent of what old leaded petrol had been. Eventually, by the late 90s, most English and European petrol stations introduced LRP - lead replacement petrol, and the problem went away. Well. Sort of......
Picture credits: Halfords and Channel 5
Lead Replacement Petrol (LRP) and valve seats
Whilst LRP solved the problem of lower octane unleaded petrol, it introduced a new problem. The lead in leaded petrol also had a secondary function and that was to lubricate the valve seats - the top of the engine block where the valves "park" when not being opened by the cams. With the advent of LRP, detonation went away but the chemicals used to increase octane didn't have any lubricating function. Some older engines started to suffer from increased wear to the valve seats, to the point where the valves could no longer properly close and seal the intake and exhaust ports. There were a couple of high profile cases before I left for America in 2001 but I've never been able to find out the end result. If you have any information on what happened in these cases, drop me a line and I'll include the info here.
The supermarket petrol debate

In England during the 90s, supermarkets started a price war with the mainstream fuel vendors by opening their own petrol stations and undercutting the Esso's and Shell's of the world by as much as 5%. People flocked to these cheap outlets without doing any proper research. There's an old saying that begins "if it's too good to be true....." In the case of supermarket petrol, there was an obvious reason why it was cheaper - it was the lower grade fuel that the mainstream outlets wouldn't take; product that had been rejected in quality control, or had less additives and detergents than what you might get from 'branded' fuel. A measurable percentage of engines started clogging up fuel lines, running badly and failing emissions tests. It was also noticed that gas mileage dropped. Eventually the supermarkets were forced to fall in line with the Big Boys, so much so that nowadays they're normally less than 1% cheaper.
Skip forwards to 2005 and the first summer of high fuel prices in America. Lo and behold, supermarkets started to sprout petrol stations and a lot of people were in the same "cheap fuel" euphoria that the English were in 10 years previously. The American market did it slightly differently though. Whereas in England, they started out with utterly sub-standard petrol, in the US it is down to the additive and detergent blends (the same system used in the UK now). All petrol vendors must have product that meets the EPA minimum guidelines and typically, branded and unbranded trucks are filled from the same supply at the refinery or distribution depot. The difference is that when a truck from one of the majors fills up, they stop at the small company pump where that company's additional detergent and additive pack is added after the main fill-up. This blend of chemicals is the 'value-added' part of the premium brand offering that makes their product meet and exceed the EPA guidelines. (The act of driving the fuel truck mixes the additive pack into the petrol). I'm not entirely clear on the percentages here, but I've spoken to tanker drivers who have claimed that it's as little has half a gallon of branded additive for each 9000 gallon truck (0.0028% additive).
There are other practices that vary between branded and unbranded petrol - for example many branded suppliers have dedicated tankers for each product - tankers that only ever carry petrol or only ever carry diesel. The unbranded suppliers have been known to save money by using the same tankers for both fuels, with a cursory 'rinse' between loads (resulting in slight cross-contamination).
This raises an interesting question then - are you better off to go branded, or to use the cheapest stuff you can find and then every couple of months, get a bottle of Chevron Techron additive (or whatever) and run that through your car?

As a substitute for genuinely cheaper fuel, a lot of supermarket chains now offer cheaper fuel at a price. The catch is that you have to shop with them. Once you buy a certain amount of stuff from their store, they'll knock off a percentage of the price of petrol if you buy it from them. The fuel isn't the cheap and nasty sub-standard stuff of yesteryear that they used to use - it's good, mainstream product. But they can hide the price drop in the cost of the groceries and other items you buy in store. From your perspective, you save £2 a tank when filling up. From the store's perspective, you just spent £100 in shopping so giving you £2 back on your tank of gas is pocket change.
In America, some of the big-box chains, like CostCo and Sam's Club do the same thing. Rather than go the "dodgy crappy petrol" route, they're offering discounted petrol for shopping in their stores, discounting the petrol by a couple of cents per gallon as long as you've bought more than $50 of products from them.
Fuel filters - without them, all this means nothing


As all this information about petrol and gasoline is starting to run out of your ears, it's worth bringing up the topic of fuel filters. Without fuel filters, none of this information on petrol is worth anything. Why? In an ideal world, every time you fill your tank, the petrol would come from brand new underground tanks, through brand new hoses and nozzles, down a pristeen filler tube into a brand new gas tank. However, back in the real world, that simply isn't the case. Tiny bits of metal flake off components. Things rust. Grit and grime gets into the fuel through many different sources. For the most part, this sediment settles at the bottom of the underground tanks in a petrol station, and at the bottom of the petrol tank in your car. If you're unlucky enough to fill up just after the petrol station has received a load of fuel from a tanker though, all that sediment will be nicely mixed into the petrol, and you'll get a petrol-sediment mix in your petrol tank. Similarly, if you insist on running your petrol tank down to the 'E' mark on the fuel gauge, you'll be sucking up petrol-sediment mix from the bottom of your own tank. It's a good job then that the men in lab coats decided to put in-line fuel filters in your car. These are relatively simple little devices that come in two basic flavours.
Carburettor engine fuel filters. These are the plastic in-line fuel filters. They look like a little plastic container with a wavy yellow pad in them. They're typically designed to have the fuel sucked through them via a mechanical crank-driven fuel pump up near the carburettor. In some tuner vehicles you'll find this has been replaced with a tasty little aluminium item, usually anodised in a nice colour, designed to make it nearly impossible to find.
Fuel injection filters. These are the metal cannister-type fuel filters that are normally buried under the car somewhere. They're designed to have the fuel pushed through them by an electric high-pressure fuel pump, and so the pressure in the fuel line is much higher. This is why they're made of metal. Internally, the filter material is normally finer too.
Why filter the fuel? Won't the debris just burn?
Generally speaking, unless it's metal filings, then yes, most debris that you'd find in a fuel system would burn during combustion but that's not the problem. The problem is getting the fuel into the engine in the first place. Further back up this page you (hopefully) learned about carburettors and fuel injectors. The one thing common to both is the tiny hole at the end of the line where the fuel is finally atomised into the air. A good sized grain of sand would be all it took to block that tiny hole and once that happens, it doesn't matter how clever your engine is, it won't be getting any petrol. That is why you have to filter fuel - to keep particulates from clogging areas of the fuel system vital to its operation.
Most manufacturers will tell you that fuel filters are sealed-for-life, or life-time-of-the-car items. Excuse my French but that's total bollocks. In normal operating conditions, in 'first-world' countries, you should change your fuel filter every 75,000 miles (120,000km) or so. If you're into extreme motoring, like round-the-world touring, or working in 'third-world' countries, your fuel filter might need changing as much as every 5,000 miles. That's not a slur on those countries, it's just a fact that the cleanliness of petrol station holding and delivery systems isn't really a hot priority in those countries. Plus, if you're involved in that sort of driving, chances are most of your petrol will come from a rusty metal jerry can.
Why change the filter?
The job of an in-line fuel filter is to filter out sediment and particulates in the petrol that might otherwise cause problems further down the line in the engine. If you think about it, the average car probably has 40 to 50 litres of petrol go through the fuel filter every week. It stands to reason then that eventually the filter is going to become clogged with debris. Once your filter gets clogged, you start to get all sorts of followon problems. In carburettor cars, you'll get sporadic and weak fuel supply which will lead to a stuttering engine, or an engine that seems to have no power under acceleration. In a fuel injection system where the fuel line pressures are much greater, a clogged filter can lead to a burned out petrol pump or a blown fuel line connection on top of the fuel starvation problems.
So just bear it in mind when you get to around 75,000 miles. If you're doing your own servicing, change the filter. If you're using a dealer, insist it gets changed despite their protests (and they will protest).
Where is my fuel filter?
You might as well ask me to explain Unified Field Theory to you here. Locating the fuel filter on any vehicle is a dark art known only to the robot that put your car together. The filter can literally be anywhere in between the tank and the engine. For carburettor engines it's most likely to be in the engine bay, probably with 50cm of where the fuel line comes up from under the car, and clipped to some other tube or cable. For injection filters, it's most likely to be attached to the chassis or a suspension component underneath the car at the back axle, close to the fuel pump and petrol tank.
The 'sock' filter.
More often than not, there will be a mesh 'sock' on the pickup tube inside the petrol tank itself. This is a much trickier filter to change as it's a sort of pre-filter to catch the really large stuff. For the most part, these mesh filters don't block easily - anything sucked up against them will normally wash off with the natural movement of the petrol in the tank. Where it's possible to change the external filters yourself, doing the internal one is probably a job best left to a decent mechanic.
The carburettor internal filter.
Some carburettors have a last line of defence in the form of a metal gause filter just inside the fuel intake. If you take the fuel line off a carburettor and peer inside, that's the most likely place for this to be if there is one. It's worth knowing about this little joy because if you go to all the trouble of changing your other in-line filter(s) and still have a fuel starvation problem, it could be this last little bugger that's blocked. Now you know.
Like the site? The page you're reading is free, but if you like what you see and feel you've learned something, a small donation to help pay down my car loan would be appreciated. Thank you.
E85 Ethanol - the magic bullet?
With the spiralling cost of fuel prices, people are looking at everything to get cheaper fuel, and one of the silver bullets seems to be E85 Ethanol-blend gasoline. I say 'seems to be' because once you do some research, which is what you're doing right here by reading this, you'll learn it's not quite the magic solution everyone would have you believe.

E85 is a blend of regular unleaded petrol with between 70% and 83% ethanol depending on the geographic location and time of year. (If you must know, Google for ASTM D 5798-99). Simply blending ethanol and petrol normally results in a product with too low a vapour pressure, especially in the winter, which is why it is a process best left to people in white labcoats in refineries.
It's designed for so-called Flexible Fuel vehicles, and as such has been classified by the US Department of Energy as an alternative fuel. The facts on E85 are a little hard to come by, so I've tried to collect together and put as many as I can right here so that you, dear reader, can try to cut to the chase. So what is a flexible fuel vehicle (FFV)? Well, it's a vehicle with an engine and emissions system designed to be able to run on a blend of unleaded petrol and ethanol up to a maximum of 85% ethanol. If E85 isn't available, you can run them on just plain old petrol though. If you read all the hoopla surrounding E85, you'll see this statement crop up time and time again: "It is a renewable source of energy and reduces the crude oil imports needed to fuel America's transportation system. Ethanol is a clean, environmentally friendly fuel.". Weeeeelllllll yes. But more specifically, "sort of". It's true that it is partly based on a renewable source of energy - ethanol is basically distilled corn oil (or wheat, barley, or potatoes. Brazil, the world's largest ethanol producer, makes the fuel from sugarcane), and yes, it's a cleaner and slightly more environmentally friendly fuel. There's a few 'buts' to go with all this, and they're a big 'buts' - of Jennifer Lopez proportions. First, there isn't enough farmland to grow enough corn to produce enough ethanol to meet gasoline demands, and it wouldn't be a good use of it even if there was. Second, there's a huge hidden cost in water - it takes 10 tons of water to process 1 ton of grain for ethanol [Ref: Plan B 2.0: Rescuing a Planet Under Stress and a Civilization in Trouble by Lester R Brown. ISBN 0393328317]. Third, in 2007, in a report on the impact of biofuels, the Organization for Economic Cooperation and Development (OECD) said biofuels may "offer a cure that is worse than the disease they seek to heal"."The current push to expand the use of biofuels is creating unsustainable tensions that will disrupt markets without generating significant environmental benefits," the OECD said. "When acidification, fertiliser use, biodiversity loss and toxicity of agricultural pesticides are taken into account, the overall environmental impacts of ethanol and biodiesel can very easily exceed those of petrol and mineral diesel," it added. And finally, in bold because it's the important part of this paragraph. E85 Ethanol-blend fuel has horrible gas mileage.
What does this mean to you? Well it means you'll need a lot more of it for a start. Sure it may be cheaper than regular petrol, but there's a reason - it's a terrible way to run a vehicle. Even the governments own figures back that statement up. Check out one of their lists of flexible-fueled vehicles for yourself. On average, putting E85 in a flexible fuel vehicle will return a nauseating 25% worse gas mileage. E85 doesn't burn as efficiently as regular petrol because it contains less energy per volume - 75,760btu per gallon as oppose to 115,400btu per gallon for plain old petrol. This accounts for the 30% increase in the amount of fuel required in the fuel-air mix during combustion, and the corresponding drop in gas mileage. All this comes with an average drop of only 10% in greenhouse gas emissions. If you go by historical precedent, and assume we all move to FFV's, the income from regular petrol will drop so the oil companies will simply increase the cost of E85. At that point, you're getting terrible gas mileage but paying what you used to for just plain vanilla unleaded petrol. Remember - nothing is free. Of course this doesn't need to be the case. E85's higher octane can allow the use of higher compression, more efficient engines (if optimized for use on it). Look at the race car teams - a lot of racing engines run on pure ethanol. And when engineered to take advantage of it, high-compression, high-efficiency engines can reduce the gas-mileage deficit to about 10% less than their petrol counterparts, which is much closer. But for ethanol to be successful it must be priced below petrol so that the cents per mile cost is favorable taking into account the drop in economy.
But what about Brazil?
For a while now, Brazil (the country, not the Terry Gilliam film) has managed to be largely independent of the world's fluctuating oil prices. By law, all Brazillian petrol must be at least 25% ethanol - E25 - created from sugar-cane-fed biorefineries. By 2007, almost all cars available in Brazil ought to be able to run on 100% ethanol. (It's worth noting that Ethanol-only cars were sold in Brazil in significant numbers between 1980 and 1995). No longer dictated-to by Big Oil, the price of their E100 is relatively low and thus it offsets the lower gas-mileage quite nicely. One argument put forth in America is that using E85 will reduce the reliance on foreign imports - specifically oil. But you need to look at the whole picture. E85 comes from corn, currently a crop used to feed people. Assuming that America has enough spare capacity to farm corn for E85 for the current demand, what happens when more people start using it? You can't increase farmland, or drop production of corn for food, so the next alternative is importing it. At which point, even using E85, you become dependent on foreign imports again. Brazil doesn't have this problem because their system is in balance and so they supply themselves with enough surplus to export their product. Most likely to America.
Clean exhaust - it depends on your definition of the word "clean".
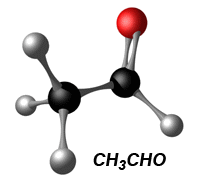
Something that isn't widely publicised is the difference in emissions between corn-based ethanol, as used in America, and sugar-based ethanol, as used in Brazil. We're all told that ethanol blend fuels produce cleaner exhaust and with sugar-based ethanol, that's absolutely true. Even with corn-based ethanol, the gasses measured at an emissions check are lower (about 25% less CO2), which still looks good. But there is something an ethanol E85 vehicle will produce through the exhaust that might surprise you. The exhaust gas contains acetaldehyde (CH3CHO) and lots of it, especially if the fuel source or combustion process is contaminated with water (like cold-start condensation). Acetaldehyde is a known carcinogen (source, source) and suspected neurotoxin (source), and when exposed to its vapors, you or I would likely develop irritation of the eyes, skin and the respiratory tract. In fact, Acetaldehyde is ranked as one of the most hazardous compounds (worst 10%) to ecosystems and human health. It's obvious why this isn't widely publicised, but then you might ask the question "why don't we see this in the emissions test?". Simple. The emissions test doesn't look for it. You can't detect and measure something you're not looking for.
But wait - it gets better. The corn-based ethanol production process consumes more fossil fuel energy than ethanol's actual calorific value. In other words, to produce a gallon of ethanol to be used in E85, it takes more fossil fuel energy than you could simply get by putting a gallon of refined non-blend petrol in your car. And as you know, regular petrol also gives better economy.
So does anyone care about this? In fact yes. The Australian government commissioned a report on this topic - you can read it for yourself here: Ethanol health impacts. The executive summary is this: ethanol does produce more of certain nasty emissions than petrol, but less of others, and that if we break everything down into dollars (the only figure the government understands) based on lost work due to illness (or death), there isn't that much difference between the two except in one area - particulate emissions (PM). PM are the nasty small pieces of carbon that can make it into your lungs, and as with smoking can (and often does) lead to cancer after a long period of time, which can then kill people. Ethanol combustion produces substantially less of this than petrol combustion (at least at 10% concentrations, and presumably more so at higher concentrations), so ends up being marginally healthier. However, it'd be healthier still to switch over to CNG, or LPG, in combination with engine improvements to make sure more of the fuel is properly combusted.
Thanks to Peter K Campbell for addition info on Ethanol and health.
E85 in non-flexible fuel vehicles
Two words : rotting seals. And I'm not talking about dead sealife. E85 is pretty acidic, and stuffing it in your regular petrol-engined car will do no end of damage to it. Apart from the spark timing and the fuel-air ratio being totally different, E85 has a whopping 105 octane rating to deal with the pre-igntion problems of having 30% more fuel in the fuel-air mix during combustion. The seals and gaskets in FFVs are designed to withstand the acidic deposits that E85 generates during combustion. Generally speaking, FFVs are manufactured to eliminate all bare aluminium, rubber and magnesium parts - all items which E85 is known to rot, and all items which a normal engine has by the bucketload. Another problem with E85 is that it's electrically conductive. Regular petrol fuel pumps aren't really designed to work with a conductive fuel, so using E85 with one could result in a fuel fire where the petrol is not only the fuel for the fire, but the electrical path for the spark. FFV fuel lines aren't made of rubber, but typically stainless steel lined with plastic.
So you may get cheaper petrol but you'll get worse gas-mileage and a broken car, with the possible bonus of a raging E85-fueled inferno to boot. However, there is a fly in the ointment here, and it is E10. Because of petrol company practices (see below), most fuel-injected engines designed and built since 1988 are already somewhat adapted to using ethanol, just not in the percentage you find in E85.
E10: you're using it right now
It's not widely known but a lot of petrol companies now blend up to 10% ethanol into their petrol products without really admitting to it, much less advertising the fact. If you've noticed your car runs somewhat less than the advertised gas mileage, that's part of the reason. Most of the gasoline in California is currently 5.7% ethanol (2% oxygen). Ethanol is blended into petrol for a variety of reasons including
- as an oxygenate to reduce CO and HC emissions
- an octane booster
- to provide volume in place of MTBE
There's nothing wrong or underhanded about this, it's just a cost effective means to legitimate ends. So if the EPA tells you you should be getting 20mpg city and you're only getting 18mpg, even driving with a feather right foot, it's not you, it's the petrol companies. 10% ethanol blend will rob you of about 5% gas mileage, and EPA figures assume a pure non-ethanol petrol. Apart from the emissions regulations, money is a factor in ethanol blending - more product that is cheaper to produce but sold for the same price. You can bet your bottom dollar (or euro) that the European refineries are doing exactly the same thing.
But what if I don't want ethanol and crappy gas mileage?
In America at least, you can go to pure-gas.org and lookup gas stations local to you that do not blend ethanol with their gas. Interestingly, the Shell station closest to where I live is a zero-ethanol station and it is consistently the cheapest branded petrol in my area. Make of that what you will.
If you know of similar sites for other countries, drop me a line and I'll add links to them.

E15: blocked by a house vote, but approved by the EPA
15% ethanol blend is getting into corrosive territory, especially for vehicles not designed with it in mind. In February 2011, the US House overwhelmingly voted to block the sale of E15 fuel in America. Representatives voted 286 to 135 against the bill that would have allowed the EPA to issue a waiver allowing petrol stations to sell E15. The renewable fuels association criticised the decision but there was almost universal support to block it from small enthusiast groups right up to SEMA (Specialty Equipment Market Association). The argument for E15 fuel was that it would help reduce the US's dependence on foreign oil. However, plenty of representatives understood the risks of allowing fuel to go sale that would only safely work in some cars sold after 2007, as well as the further drop in fuel efficiency that comes with a 50% higher ethanol content.
However in April 2012, the EPA disregarded all objections and pushed ahead to approve the use of E15 blended petrol despite admitting that it will cause problems in vehicles manufactured before 2001. (In reality, that restriction applies to any vehicle built before 2007). This will be a problem for petrol stations as they will need to provide both E10 and E15 fuel for their customers, meaning the installation and maintenance of new underground tanks and above-ground blender pumps. That will be expensive and there's a good chance that they'll either skip E15 altogether or simply stop selling E10 and screw the motoring public.
The EPA's move was for sure coupled to the ending of the 30-year tax subsidy on corn-based ethanol and the cessation of tariffs on ethanol imported from Brazil. The move itself prompted many manufacturers to indicate that if you use E15 blend, it will void your factory warranty. The battle continues.
Tax credits, subsidies and tariffs - the real story behind E85.
So given the (obscured) facts about corn-based ethanol, why the big push in America to go to E85? Simple. Money. The government is offering tax credits to the big car manufacturers to produce FFVs, even if none of them ever run on E85. Similarly, tax credits are now offered to the big oil companies to product E85 ethanol blend, even if they don't actually sell it. And when they do sell it, it will make the more money because you and I will need more of it to go the same distance. Finally, corn growers receive federal subsidies for growing corn for ethanol production. Couple that with the 54¢ per gallon tariff that is currently levied on Brazillian imports, and it shows how the corn-based ethanol has cornered the American market and is keeping the cheaper, cleaner, sugar-cane ethanol at bay.
What's that you say? You want fuel efficiency and less cost?
Picture credit: Volkswagen

Well that's the conundrum isn't it? The oil and car companies aren't going to give stuff away free. So you have to choose. Do you want less cost at the point where you're putting the petrol into your car, or less cost-of-ownership? It's like comparing financial planning. If you get a flexible fuel vehicle, your immediate cost is much less - you could be spending 30% less per fill-up. But the long-term costs become negligible because of the bad gas mileage. On the other hand, if you take the long term investment point of view, you should be looking at vehicles like the VW Polo Bluemotion. It's a three-cylinder turbodiesel, which means at the point of filling it up, you'll actually be spending more than a regular petrol vehicle. But it returns 70mpg (max), so you'll be visiting the petrol station a lot less frequently. Don't understand the maths? Ok, lets lay it out.
I'm going to assume plain fuel costs here, so I'm not factoring in insurance, wear and tear, initial cost of the vehicle etc. Ready? Okay, we're going to compare two vehicles. Each drives 15,000 miles a year and each has a 16 gallon fuel tank. The owners of these vehicles are Barbie and Ken, and to be suitably sexist, Barbie has a pink VW Polo and Ken has a blue Ford Crown Victoria. They both fill up when the tank gets to 3 gallons left, so they drive 13 gallons at a time.

Ken
15,000 miles = 1250 gallons at 12mpg on E85.
1250 gallons = about $2300 assuming about $1.85 a gallon.
Ken stops and fills up every 156 miles.

Barbie
15,000 miles = 225 gallons at 66.5mpg (split the values of 60mpg and 73mpg for city and highway) on diesel.
225 gallons = about $787 assuming about $3.50 a gallon.
Barbie stops and fills up every 864 miles.
So whilst Ken pays much less each time he fills up, he's filling up nearly 6 times as often, and at the end of the year, he's spent a whopping $1500 more in fuel costs on this nice, 'clean, environmentally-friendly' E85 ethanol. Now I don't know about you, but it seems to me that the pollution from 225 gallons of diesel is going to be a whole hell of a lot less than the pollution from 1250 gallons of E85.
Obviously this example is extreme, but it does use real-world facts and figures from real-world vehicles you can buy right now. I did it to illustrate how being in posession of the facts can help clear up the doublespeak and misinformation. So if you're considering an E85-fuelled vehicle, you might want to do some more homework first, because it most certainly is not the silver bullet we're all being led to believe.
For more information / propaganda, go to the official E85 fuel site.
LPG / LNG / AutoGas - Liquid Petroleum Gas / Liquid Natural Gas

In Europe, LPG has been an option for drivers for years. In Australia, it's been around since the 60's. In England, it's still bubbling under and in America it's virtually unheard of. So what is it? Well, put simply, it's petroleum or natural gas compressed to the point where it becomes a liquid. The liquified gas is contained in a pressure vessel inside the car somewhere - normally a tank in the boot (or trunk if you're American). There's a feed line from there to the fuel injection system or carburettor and a solenoid switch connected to a fuel-cutoff switch, both of which are controlled from a button or switch on the dashboard. In one position, the LPG line is closed and the petrol line is open and the car works just like every other car on the road. In the other position, the petrol line is cut off and the LPG line is opened up. Liquid gas under pressure shoots up the line and out of an injector nozzle either screwed into the side of the carburettor, or integrated into the fuel injection system. As the gas expands out of the nozzle it cools down and becomes a gas. The gas is highly combustible and when mixed with the air going into the engine, creates a perfectly useable fuel-air mix for the engine to run on. Simple.
The gas itself is normally some derivative of butane or propane, or a combination of the two. LPG is manufactured during the process or refining crude oil, and LNG is manufactured during the refining process of extracting natural gas from the ground. Once the gas is compressed, it becomes a liquid, and this is what is carried around in the tank in the back of your car.
The pros and cons of LPG
LPG is popular because for the most part, it's cheaper than petrol and it gives a pretty good gas-mileage. There are three key issues that bother most people considering LPG conversions. The first is the tank - it takes up a lot of space in your car. For some this isn't an issue, but for others, they need the space and they don't like the idea of an ugly pressure tank up on the roof of the car. The second issue is availability. On mainland Europe this isn't a problem but in most of the rest of the world, you'd be more likely to win the lottery than just stumble across an LPG filling station. The good news though is that by flicking the switch on the dashboard, you can go back to regular petrol (as long as you've got some in the tank) and keep filling up like that until you do come across another LPG station. The third issue is cost. It costs a fair amount to get an LPG conversion done. LPG is cheaper and in the long run you'll recover the costs and start saving money, but that can take 50,000km or more. Another issue which some people don't like is the idea of the pressurisation system. To fill up an LPG tank, you need to hook on a pressurised hose to a special filler cap on the outside of the car. It's easy enough to do, and it holds itself in place whilst you're refuelling, but for some, they just don't like it. The tank itself will normally be fitted with an automatic fill limiter which looks a lot like a toilet bowl float. When the tank is nearly full, the float operates a lever which severely restricts the flow of gas into the tank. This causes enough backpressure for the pump to realise that it's time to cut off the flow.
Hydrogen
Hydrogen has been touted as the Next Big Thing in terms of eco-friendly fuels for our cars but is it? Hydrogen can be used either in internal-combustion form similar to burning petrol, or as a fuel source for a fuel cell that generates electricity for electric drive motors. Currently both cases are a bit sketchy for mainstream transport. Future developments will change that but why is it currently unsuitable?
The two main reasons are the cost of production and the problems with storing it once refined.
Hydrogen does not exist as a source of energy without refining. It needs to be extracted from something else in order to be used. This extraction process can be costly and dirty, resulting in energy losses between 15% and 50% during extraction and compression. In the case of using hydrocarbons as the source, the resulting hydrogen is more polluting than petrol or diesel once burned. The more likely method of extracting hydrogen is using water as the source and electrolysis as the method but that is both more expensive and slower. Whilst it's true that for the most part the only exhaust from a hydrogen vehicle is water, the problem is that the energy required and pollutants released during the processes described above are no more appealling than the processes used in refining petrol.
To store refined hydrogen there are two options - compressed or liquified.
Compressed hydrogen occupies three times the volume of petrol for the same energy. It's normally compressed to something like 12,000psi although doing so is technically difficult and consumes a considerable amount of energy. Containing that pressure in a tank is also difficult; a steel tank would weigh one hundred times the weight of the hydrogen it contained which is impractical. A carbon fibre tank will do the trick but to contain that pressure, the cost of the tank becomes prohibitive. It's also worth noting that if a 12,000psi tank of hydrogen ruptured (for example in a collision) it would release the same amount of energy as the same weight of dynamite.
Liquified hydrogen, as with compressed hydrogen, also occupuies three times the volume of petrol for the same energy. (Oddly, there's more hydrogen in a gallon of petrol than in a gallon of liquid hydrogen). Unlike the compressed version, tanks to contain liquid hydrogen are considerably lighter but must be extremely well insulated or actively cooled. They're like a thermos flask inside another thermos flask so they're quite delicate. Unfortunately because of its physical properties, hydrogen is one of the hardest gasses to liquify, resulting in energy losses between 30% and 50% in the liquification process.
On top of all that, hydrogen molecules are very small and can actually leak through the walls of a steel tank. Kawasaki performed an experiment in Japan where they drove a truck full of cooled hydrogen around and lost about 6% of the payload through leakage. The hydrogen industry in general reckons that an average tank will lose 5% per day through this process.

Because of all the above, the cost of the vehicles is currently prohibitive for all but the developers. In 2010 all the development vehicles were over $1,000,000 a piece. There were only 20 hydrogen fueled cars in America and even less in Europe and Asia. Arnold Schwarzenegger's Hydrogen Hummer was a one-off $1,200,000 vehicle and wasn't representative of anything a consumer could buy.
Assuming you could own a hydrogen vehicle, you then face the lack of infrastructure. California's much-touted Hydrogen Highway is still a pipedream likely to be mired in government debate forever because of the cost. Getting hydrogen to the filling stations is also a problem. A typical petrol tanker can deliver 25 tons of petrol but only 400Kg of hydrogen because of the tank requirements. So a hydrogen filling station would need 15 times the number of deliveries per day to keep it in business. Consider now the commensurate cost in fuel consumed and pollution released to deliver all that hydrogen. So to the consumer, the sticker shock would be extreme. All that expense in refining, transportation and storage would be passed on to the consumer.
That's assuming you could reach the filling station in the first place. Remember compressed hydrogen occupies three times the volume of petrol of the same energy, so you'd need a tank three times the size of a petrol tank to get the same range. The current demonstrator vehicles have hydrogen tanks that fill most of the trunk and a lot of the rear passenger space, and can barely squeeze out 100 miles between fill ups. The GM and Toyota hydrogen demonstrators are always trailered to their demonstrations simply because they would never get there if they were actually driven. The highest range to date from a hydrogen vehicle is BMW's hydrogen demonstrator that managed 240 miles.
The fuel cells for hydrogen-electric cars are another problem yet to be overcome. Whilst they might increase the paltry range further, you need a lot of fuel cells to produce a usable amount of electricity. Stack enough together and they have the equivalent weight of the same number of batteries required to make a pure-electric vehicle but, with the overhead of the pure cost of fuel cells. The fuel cells themselves are expensive to produce and very fragile. They don't take well to bumps and jolts - the environment they'd be exposed to in a vehicle - and they require relatively large amounts of relatively rare substances like platinum to make their catalysts.
Then there's that whole Hindenburg thing. Sure, the hydrogen didn't cause the fire, but it fueled it. Imagine now crashing in a vehicle with fragile fuel cells, plumbing lines and pressurised tanks full of hydrogen. The manufacturers are keen to point out that their systems are super safe which is a fair comment but what about the 1 in 1000 crash that will happen every now and then if the vehicles are delivered to the public? What about the fact that hydrogen can't just be vented to the atmosphere because it's an explosion hazard?
Picture credit: BMW Press Kit
The "Run your car on water" brigade - HHO
Let me start this section with the following statement: it takes more energy to get hydrogen out of water than you can ever get back by burning hydrogen in an engine. There's just no way around that energy can't be created or destroyed, but it can be wasted, usually as heat. Getting hydrogen from electrolysis certainly does that, being only 50-80% efficient at best. Those are the laws of thermodynamics and conservation of energy, and no amount of clever marketing, lawsuits, doublespeak or clever words is going to change that. With that in mind then:
You might have come across ads on the web - maybe even ones that Google have fed to this very page - claiming you can buy a kit to make your own car run on water. These kits use high school physics to do it relying on simple electrolysis - the process of using electric current to dissociate water molecules. The resulting gas is oxyhydrogen (sometimes called Brown's Gas or HHO). It's what is used in some cutting torches. With a little fuel-line plumbing and some basic handyman skills, you can fit such a device to any engine, fill the extra tank with water, and be generating hydrogen in no time. The gas is fed into the combustion chamber along with your normal fuel-air mix and sometimes you'll see better gas mileage. However, you're using a lot of electricity from the car's electrical system to generate the hydrogen, increasing the load on the alternator, meaning more drag on the engine to turn the alternator, meaning a less efficient engine.
And that right there is the divisive argument that splits the car camp in half. Some people are also so convinced that the internal combustion engine is so efficient that nothing we can do that would increase power would make up for this reduction, and as such refuse to even consider the possibilities. They find it difficult to get past the losses introduced by the electrolysis of water into hydrogen as stated above. But what about the improved efficiency in the combustion of the liquid fuel this hydrogen can cause? Look back to the video at the top of this page. During combustion, the fuel burns rather than explodes in the cylinder, which means that quite a bit of the energy is released after the piston has already accelerated downwards, or even when it reaches the bottom. As such, modern internal combustion engines are extremely inefficient at converting liquid fuels to usable power. This is why the introduction of a gaseous fuel with a much higher flame front (like hydrogen) can make a difference in fuel economy. Hydrogen's flame front speed is so high it effectively does explode, so the addition of just a few percent of it in the mix ensures that virtually all of the liquid fuel's energy is actually turned into power - a short, sharp "bang" (relative to the speed of the regular ignition) that pushes the piston down with great force, dramatically reducing emissions in the process.
So if this glorified high school experiment really works, why hasn't it been taken up by the car manufacturers? Unfortunately you won't see this kind of radical improvement on modern vehicles (at least without substantial additional modification) due to fuel injection and the ECU. You can learn elsewhere on this page that the ECU has maps set up to ensure the car works in a certain fashion. When you introduce extra oxygen into the tailpipe (either with the hydrogen when produced by electrolysis, or because of a more efficient combustion process) the computer thinks that you're running too lean and hence increases the amount of fuel going into the vehicle - so you end up with more power, but not necessarily a better fuel consumption. For HHO systems to work in practice generally requires older carburettor engines and a serious modding of the whole electrical system (and ECU on more modern vehicles) (see chipping and remapping). In addition, an efficient hydrogen electrolysis system itself costs a few hundred dollars to construct. Naturally that means there are plenty of dodgy operators out there on the internet selling cheap systems that have little or no effect in your vehicle in the real world. For a true hydrogen generator setup, you need deep pockets. If you want to read more on this topic, there are papers available for purchase, most notably the one by researchers at a Turkish university who showed that HHO generated on-the-fly using a vehicle's own power source, improved efficiency of combustion in 4 carburettor vehicles (very important to note that point) by so much that overall fuel usage dropped by up to 40%. That paper was published in the International Journal of Hydrogen Energy in 2000 and is available here: Fuel economy improvement by on board electrolytic hydrogen production.
Thanks to Peter K Campbell for pointing me in the right direction on this topic.
Let the lawsuits and complaints begin....
In August 2009, the first complaint against a company promoting a consumer-level HHO system as being able to make your engine run further, cooler, and for less cost was filed and upheld in England. Their ad was deemed to be misleading on 4 counts (and it claimed that their system could turn your car into a petrol-hydrogen hybrid). The full text of the adjudication should be searchable on the ASA website: Waterboost Hydrogen Fuel System complaint.
The slightly more 'out there' version of running your car on water

No article on water powered cars would be complete without mentioning Stan Meyer or any number of other people who claim to have made cars run on water alone. Stan Meyer's idea was more unique than most - rather than generating hydrogen and storing it to be burned later, he claimed his system was a water splitter that fractured water into hydrogen and oxygen instantaneously to be burned. The water never got hot and he claimed it wasn't pure electrolysis because he was using very low current. He spent a lot of time selling the idea and getting patents for a system of electronics and oscillating radio frequencies coupled to his separation chamber. It sounded too good to be true. When he started telling the press that he'd been offered $1bn by the Arabs to sit on his invention, and that he was getting 1700% more energy out than he was putting in, it looked more and more like a scam. I believe Meyer was eventually convicted of fraud for all this in 1996 and then died in mysterious circumstances in 1998. Conspiracy theorists will tell you that he was seen off by Big Oil.
The problem is that you have to remember other huge 'scientific breakthroughs' in this area, like like Martin Fleischmann and Stanley Pons claiming to have solved cold fusion. Something else that seemed to be too good to be true and in the end turned out to be just that. I've got to stick to current scientific thinking on this for the time being, that being the law of conservation of energy again. Until someone can publicly demonstrate such a system that anyone can reproduce, and reproduce successfully and frequently, Meyer's water fracture device will remain a mystery.
The eBay problem
This paragraph may seem a little out of place but I have had a lot of problems with a couple of eBay members (megamanuals and lowhondaprelude) stealing my work, turning it into PDF files and selling it on eBay. Generally, idiots like this do a copy/paste job so they won't notice this paragraph here. If you're reading this and you bought this page anywhere other than from my website at car-bibles.com, then you have a pirated, copyright-infringing copy. Please send me an email as I am building a case file against the people doing this. Go to car-bibles.com to see the full site and find my contact details. And now, back to the meat of the subject....
